Frequently Asked Questions of Clinical Research
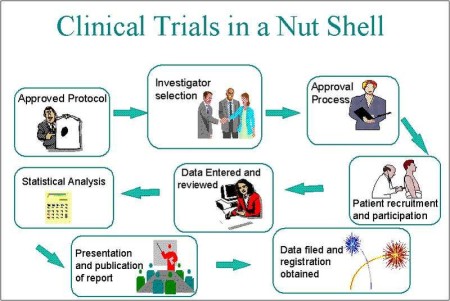
What are clinical trials?
A research study involving human volunteers. as a volunteer, you may discontinue a study at any time for any reason. also you may be discontinued from a study for safety reasons.
How am I protected as a volunteer?
U.S Federal Agencies including the FDA (Food and Drug Administration) and the NIH (National Institutes of Health) oversee many of the studies conducted in the U.S. Also IRB's (Institutional Review Board) review and approve study protocols to ensure that a study is ethical and protects the volunteers rights.
Why do some participants receive a placebo?
Placebos are often used in clinical research studies to help researchers determine how effective the investigational drug is. Without a placebo, it’s very difficult to determine whether any changes during the study are due to the investigational drug or some other factor. Placebo-controlled studies are usually ‘double-blind’. This means that neither the study doctor nor the participant know whether they are receiving the investigational drug or a placebo.
Do I need insurance to participate in a clinical trial?
No insurance is needed. In many instances you may be compensated for your time and travel.
Will I be seen by a physician?
In almost all studies exams by a physician associated with the study are required for study enrollment. There is no fee for this. all study procedures are completed on site, in our office, and free of charge. We may also require previous medical records related to your disease or illness but will help you facilitate that as well.
If you have any other questions please feel free to email or call us.
studies@mctrials.net
703 527 8100